Dr. Daw Hnin Hnin Than
ဒေါက်တာ ဒေါ်နှင်းနှင်းသန်း
Position : Professor (Head)
Degree : B.Sc (Honours), M.Sc(Nuclear Chemistry), Ph.D (Nuclear Chemistry)
Other Information : Diploma in Global English
Chemistry
Position : Professor
Degree : B.Sc (Honours), M.Sc(Inorganic Chemistry), Ph.D ( Inorganic Chemistry )
Other Information Diploma in English(SEMEO), DCSC.(Computer) Position : Professor
Education : B.Sc (Hons:), M.Sc (Chemistry), Ph.D (Chemistry)
Teacher list
| Sr.No | Department | Name | Position | Education | Thesis Title | Field Of Specialization | Current Research Project | Email/Gmail |
| 1 | Chemistry | Dr.Hnin Hnin Than | Professor (Head) | Ph.D | Elemental Analysis and Biochemical Studies on Traditional Medicine Formulation Hseezaybyu | Inorganic and Nuclear Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application | [email protected] |
| 2 | Chemistry | Dr.Mya Thu Zar | Professor | Ph.D | Carbon Molecular Sieves As Intercalation Catalytic Support | Inorganic Chemistry | [email protected] | |
| 3 | Chemistry | Dr.Ohnmar Tin | Professor | Ph.D | Immobolization of Isolated Laccase from Thitis and Its Enzymatic Reaction with Some Phenolic Compound | Physical Chemistry & Enzymology |
[email protected] | |
| 4 | Chemistry | Dr. Soe Soe Naing | Professor | Ph.D | Isolation and identification of Natural Compounds from Shinmataung Thanakha ( hesperethusa crenulata Roxb.) and Antimicrobial activity of Different Solvent Extracts and The Isolated Compounds | Analytical Chemistry | [email protected] | |
| 5 | Chemistry | Dr.Kyi Kyi Than | Associate Professor | Ph.D | Some Aspect of the Conductivities of Hydrochloric Acid Doped Polyaniline | Physical Chemistry | [email protected] | |
| 6 | Chemistry | Dr.San San Mon | Associate Professor | Ph.D | A study on optimum conditions of fermentation for production of vinegar from grape | Organic Chemistry | [email protected] | |
| 7 | Chemistry | Dr.Mya Mya Sainn | Associate Professor | Ph.D | Investigation on Cellulose Fiber (polysaccharide) from Coconut White Meat For Enzyme Immobolization | Organic Chemistry& Enzymology | Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh | [email protected] |
| 8 | Chemistry | Dr. Kay Khine Nyunt | Associate Professor | Ph.D | Studies on the Radical Scavenging Activity and Effect of Some Isolated Compounds from Glycine max Linn. (Soybean) and Extracts from Panax zingiberensis Linn. (Ginseng) on Isolated Uterine Smooth Muscle | Organic Chemistry | [email protected] | |
| 9 | Chemistry | Daw Moh Moh Cho | Lecturer | M.Sc | Study on Caffeine Content in Commercially Available Different Brands of Instant Coffee | Organic Chemistry | mohmohcho2012@gmail. com |
|
| 10 | Chemistry | Daw Aye Aye Maw | Lecturer | M.Sc | Electroless Alloy Plating of Ni-Cr on Various Plastics | Physical Chemistry | [email protected] | |
| 11 | Chemistry | Dr. Aye Sandar Shwe | Lecturer | Ph.D | Synthesis,Characterization and Application of Nickel Aluminium Nitrate Layered Double Hydroxide Nanocomposite | Inorganic Chemistry | sandarshwe1364@ gmail. com |
|
| 12 | Chemistry | Dr.Tin Mar Aung | Lecturer | Ph.D | Performance Studies On Mesophilic And Thermophilic Anaerobic Digestion Method For The Treatment Of Wastewater From Fermentation Process | Physical Chemistry | dr.tinmaraung @ gmail.com | |
| 13 | Chemistry | Dr.Thin Thin Myaing | Lecturer | Ph.D | Studies On The Purification And Regeneration Of Used Railway Engine Oils By Using Activated China Clay | Physical Chemistry | thinthinmyaing477@gmail | |
| 14 | Chemistry | Dr.Mar Mar khine | Lecturer | Ph.D | The Effect of Neutron Radiation on Pedisein Seeds (Vigna radiata L. Wilczek) for Cultivation | Nuclear Chemistry | [email protected] | |
| 15 | Chemistry | Dr.Satanar Tun | Lecturer | Ph.D | Synthesis, Characterization and Application of Aluminum Oxide and Magnesium Aluminate Nanoparties | Analytical Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application | decembersaytanar@gmail. com |
| 16 | Chemistry | Dr.Hnin Htet Wai Nyunt | Lecturer | Ph.D | Investigation of Some Bioactive Constituents and Bioactivity of Leaves and Flowers of Melastoma malabathricum L. (Nyaung-ye-o-pan) | Organic Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application | hninhtetwainyunt.chem@ gmail.com |
| 17 | Chemistry | Daw Phyu Phyu Zaw | Lecturer | Ph.D | Study on some Dermatological Properties and Chemical constitient of Myanma Natual Cosmastic (Ferronia limonia L.swingal (Thee bark) | Organic Chemistry | [email protected] | |
| 18 | Chemistry | Dr. Daw Nan Yin Yin Htwe | Lecturer | Ph.D | Enzymic Properties of Arginase Enzyme from Embryonic Axes of Soybean (Glycine max. L.) and its Application in Clinical Analysis | Analytical Chemistry & Enzymology | [email protected] | |
| 19 | Chemistry | Dr.Khin Theingi Toe | Lecturer | Ph.D | Seasonal Variation in Quality of Coastal Sea Water around Ma Gyi Area, Ayeyawady Region in Rakhine Coastal Zone, Myanmar | Analytical Chemistry | [email protected] | |
| 20 | Chemistry | Dr. Nang Htay Htay Win | Lecturer | Ph.D | Structural Elucidation of Secondary Metabolites From Dried Leaves of Laphet from Pindaya Township and Processing of Cosmetic From Tea Leaves | Organic Chemistry | nanghtaywin@gmail. com |
|
| 21 | Chemistry | Dr. Su Myat Htay | Lecturer | Ph.D | Synthesis,Characterization and Application of Graphene Oxide(GO) and reduced Graphene Oxide(rGO) | Analytical Chemistry | sumyatchem852018@gmail. com |
|
| 22 | Chemistry | Daw Phyu Phyu Myint | Lecturer | M.Res | Synthesis,Characterization and Application of ZnO-ZnS Core-Shell Nanoparticles | Analytical Chemistry | phyuphyumyint746@gmail. com |
|
| 23 | Chemistry | Dr. DawToe Thiri Toe | Assistant Lecturer | Ph.D | Comparative Study on Betulinic Acid Contents in the Barks of Tectona Hamiltoniana Wall. (Da-Hat) grown in different region of Myanmar and Investigation of some bioactivities | Organic Chemistry | [email protected] | |
| 24 | Chemistry | Daw Nway Oo Lwin | Assistant Lecturer | M.Res | Study on physicochemical properties of (Star fruit) Averrhoa Carambola L. | Physical Chemistry | nwayoolwin 3290@gmail. com |
|
| 25 | Chemistry | Daw Su Myat Aung | Demonstrater | M.Sc | Study on the activity of α- glucosidase from rice (Zeeyar) | Analytical Chemistry & Enzymology |
An Experimental Study on the Recycling of Banana Peels of Environmental Application Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh |
[email protected] |
| 26 | Chemistry | Daw Yatanar Aye Thant | Demonstrater | M.Sc | Investigation of Chemical Constituents and Bioactivities of Essential Oil Extracted from the Bulbs of ALLIUM SATIVUM LINN. (Garlic) |
Organic Chemistry | Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh | yatanarayethant123@gmail. com |
| 27 | Chemistry | Daw Soe Thandar Tun | Demonstrater | M.Sc | A Study On The Nature Of Ascorbic Acid (Vitamin C) Extracted From CARICA Papaya L. (Papaya Fruit) Under Different Conditions | Organic Chemistry | soethandartun.doedoe@gmail. com |
|
| 28 | Chemistry | Daw Pyae Pyae Phyo | Demonstrater | M.Sc | Screening of Phytochemical Constituents and Some Bioactivities of the Leaves of Murraya koenigii L.(Pyim-daw-thein) | Organic Chemistry | pyaepyaephyo418@gmail. com |
|
| 29 | Chemistry | Daw Su Lae Nandar | Demonstrater | M.Sc | Study on Some Chemical Constituents and Bioactivity from the bark of Holarrhena Antidysenterica Wall.(Let tok gyi) | Organic Chemistry | [email protected] | |
| 30 | Chemistry | Daw Thet Thet Aung | Demonstrater | M.Res | Water Quality Assessment of Nga Moeyeik Creek Water | Analytical Chemistry | [email protected] | |
| 31 | Chemistry | Daw Khaing Su Wai | Demonstrater | M.Sc | Preparation and Characterization of Some Snacks from Black Gram(Mat Pe) and Chick Pea(Kala Pe) Samples | Analytical Chemistry | Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh | [email protected] |
| 32 | Chemistry | Daw Hnin Lae Thwe | Demonstrater | M.Sc | Study on the enzymatic properties of alpha amylase from Cassava (Manihot escunleta Crantz) | Analytical Chemistry | [email protected] | |
| 33 | Chemistry | Daw Hla Moe Swe | Demonstrater | M.Res | Investigation of Arsenic Contents and Antimicrobial Activities of Some Selected Myanmar Traditional Medicine Formulations | Inorganic Chemistry | [email protected] | |
| 34 | Chemistry | Daw Myat Myint Zu | Demonstrater | M.Sc | Extraction, Characterization and Application of Natural Dye from Persea Americana Mill. (Htaw Bat Thee) Seed | Organic Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application | [email protected] |
| 35 | Chemistry | Daw Yamone Pyae Pyae Thin | Demonstrater | M.Sc | Investigation of Phytochemical Constituents and Some Biological Activities of Bergenia Ciliata (Haw).Sternb (Nat-Sein-Gamone) | Organic Chemistry | yamonpyaepyaethinn88@ gmail.com |
|
| 36 | Chemistry | Daw Khin Po Po Kyaw | Demonstrater | M.Sc | Nutritional Values and Screening of Phytochemical Constituent of Three Kind of Defatted Peanut Seeds | Analytical Chemistry | [email protected] | |
| 37 | Chemistry | Daw Eaindra Su Hlaing | Demonstrater | M.Sc | Study on the preparation and biodegradation of potato starch film | Physical Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh |
eaindrasuhlaing [email protected] |
| 38 | Chemistry | Daw Nan Kalyar Chit | Demonstrater | M.Sc | Extraction and Characterization of Starch from the Tubers of Dioscorea alata L. and its Application in Rice Substituted materials Production | Organic Chemistry | Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh | [email protected] |
| 39 | Chemistry | Daw Ei Nandar Aung | Demonstrater | M.Sc | Extraction and Characterization of Natural Food Dye from Fruits of Borassus | Physical Chemistry | [email protected] | |
| 40 | Chemistry | Daw Ahthin Khayar | Demonstrater | M.Sc | Study on the Biodegradation of Prepared Cassava Starch Film | Physical Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh |
[email protected] |
| 41 | Chemistry | U Zaw Min Maw | Demonstrater | M.Sc | Chemical Composition of Fruit Pulp in Dragon Ftuit (Nagamauk) | Analytical Chemistry | [email protected] | |
| 42 | Chemistry | Daw Thu Thu Lwin | Demonstrater | M.Sc | Investigation on Chemical Constituent in Essential Oil from Lemongrass (Cymbopogon citratus) | Organic Chemistry | [email protected] | |
| 43 | Chemistry | Daw Ei Ei Mon | Demonstrater | M.Sc | Study on the removal of heavy metal Ions by prepared cellulose Acetate-rice husk composite films | Physical Chemistry | An Experimental Study on the Recycling of Banana Peels of Environmental Application | [email protected] |
| 44 | Chemistry | Daw Htay Yee Win | Demonstrater | M.Sc | Screening of Phytochemical Constituents and Some Biological ativities of Melastoma malabathricum L. (Oboke- Gyi )Leaf | Organic Chemistry | [email protected] | |
| 45 | Chemistry | Daw Myat Khin Zar Tun | Demonstrater | M.Sc | Investigation of Preliminary Phytochemical Constituents and Some Bioactivities from the Leaf and Stem of Coccinia grandis (L.) (Kinbon) | Organic Chemistry | myatkhinzartun321k@gmail. com |
|
| 46 | Chemistry | Daw Than Than Htway | Demonstrater | M.Sc | Effect of Deep Fat Frying on Chemical Properties of Palm Oil Elaeis Guineensis jacq. | Analytical Chemistry | thanthan34679087@gmail. com |
|
| 47 | Chemistry | U Soe Yan Naing Win | Demonstrater | M.Sc | Investigation of Contaminated Level of Sediments from the Flooded Areas of Mrauk U Township, Rakhine State | Analytical Chemistry | soeyannaingwin30@gmail. com |
|
| 48 | Chemistry | Daw Myat Su Mon | Demonstrater | M.Sc | Investigation of Antioxidant and Antimicrobial Activities of Red Sesbania Grandiflora (L.)Pers. (Paul-Pan-Ni)Flowers | Organic Chemistry | [email protected] | |
| 49 | Chemistry | Daw Khin Zar Zar Moe | Demonstrater | M.Sc | Effects Of Natural And Synthetic Mordants On Cotton Dyeing Using Waste Skin Of Onion (Allium Cepa L.) | Analytical Chemistry | [email protected] | |
| 50 | Chemistry | Daw Minn Minn Thaw | Demonstrater | M.Sc | Electrolytic Preparation of Metallic Copper Nanoparticles from Aqueous Copper (II) Sulphate Solution and its Antimicrobial Activity | Physical Chemistry | [email protected] | |
| 51 | Chemistry | Daw Khin Thandar Myint | Demonstrater | M.Sc | Studies on the Stability Constant of Complex | Analytical Chemistry | [email protected] | |
| 52 | Chemistry | Daw Thel Su Ye Tun | Demonstrater | M.Sc | Biosynthesis and Characterization of Iron Nanoparticles from Extract of Azadirachta Indica (Neem) Leaf for Antimicrobial Activity | Physical Chemistry | [email protected] | |
| 53 | Chemistry | Daw Phoo Pwint Phyu Hnin Than | Demonstrater | M.Sc | Biosynthesis and Characterization of Copper Nanoparticles Using Azadirachta Indica (Neem) Leaf extract and its Antimicrobial Activity | Physical Chemistry | [email protected] | |
| 54 | Chemistry | Daw May Thu Aung | Demonstrater | M.Sc | A Study on some Activities of Various Crude Extracts and Essential Oil from Rhizomes of Zingiber Officinale Rosc. Gyin) | Organic Chemistry | Extraction and Application of Oils from Waste Cocos nucifera L. (Coconut) White Flesh | [email protected] |
Programmes Offered
- B.Sc. / B.Sc. (Hons) in Chemistry
- B.Sc. / B.Sc. (Hons) in Biochemistry
- M.Sc. and M.Res. in Chemistry
Curriculum
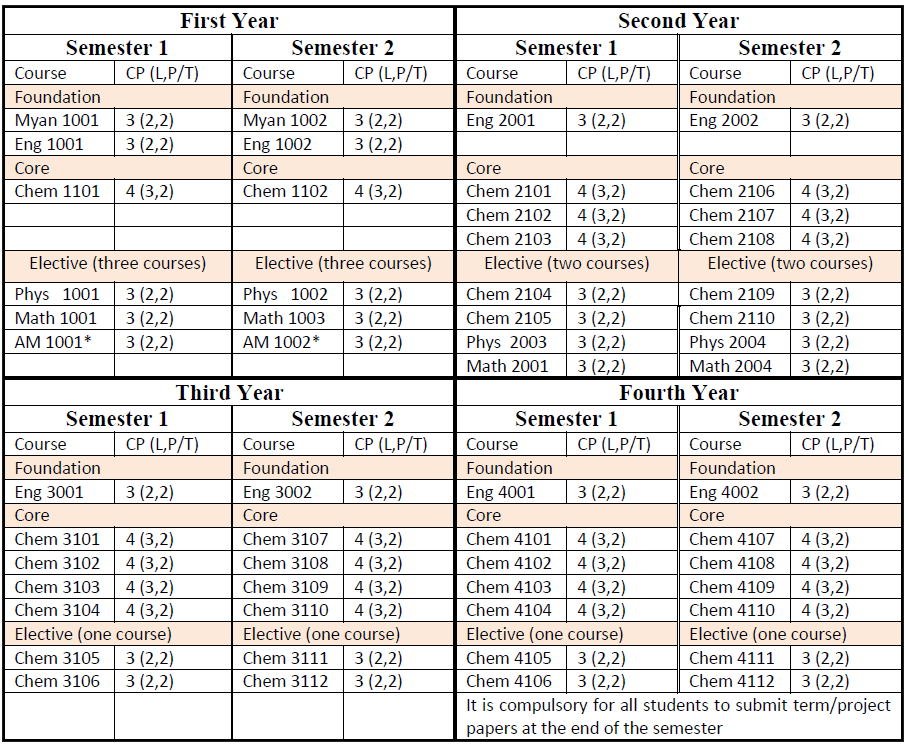
B.Sc. in Chemistry
B.Sc. (Honours) in Chemistry
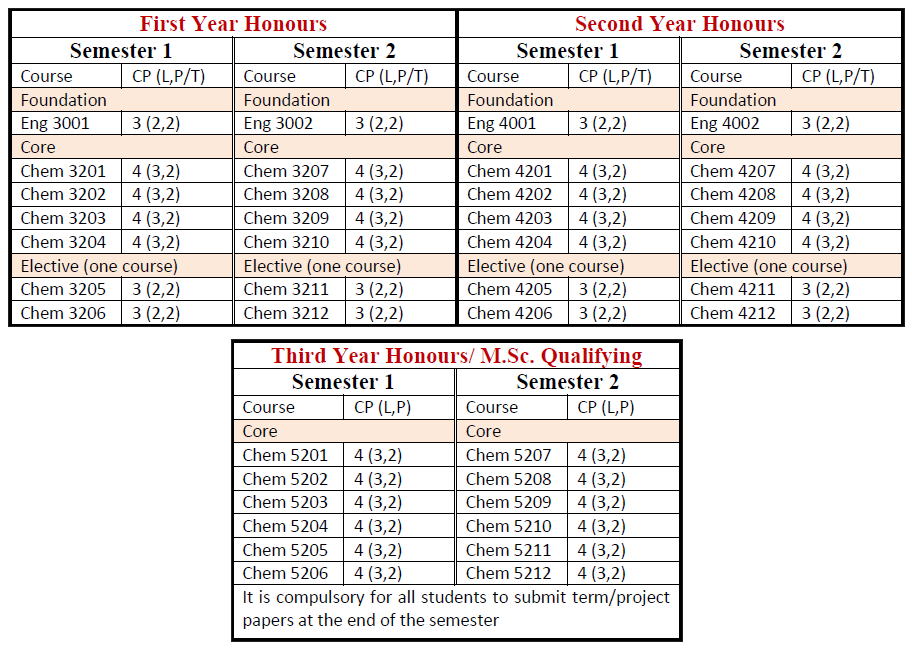
Students who passed second year with GPA ≥ 4 are eligible to attend B.Sc. (Honours) classes for three more years. After finished successfully, they earn B.Sc. (Hons) degree majoring in Chemistry.
M.Sc. in Chemistry
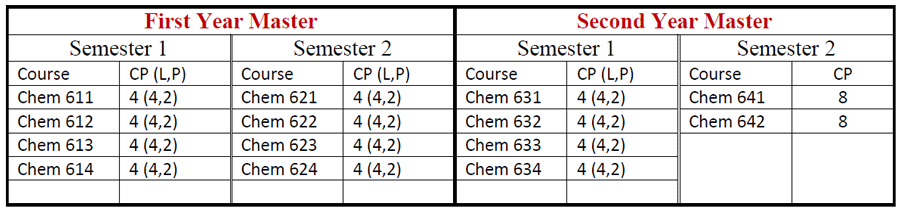
Students who passed B.Sc. (Honours) with GPA greater than 4 are eligible to attend M.Sc. classes for two more years. After finished successfully, they are earned M.Sc. degree majoring in Chemistry.
Course Descriptions
In second semester, students will learn chemical bonds, metals and metalloids, general properties of aqueous solutions and chemistry of some aliphatic compounds such as ethers, aldehydes and ketones.
In second semester, students will learn the properties of gases, the properties of solutions and the chemistry of aliphatic carboxylic acids (monocarboxylic acids) and functional derivatives of carboxylic acids (esters & amides) and amines (monoamines).
In first semester, students will learn three main parts. The first part deals with transition metals that include members and configurations, characteristic properties, oxidation states and compound formation, 3d-, 4d- and 5d-transition series elements, complex ion formation, chromium, manganese, iron, cobalt and nickel. Second part is concerned with basic concepts of thermodynamics that encompass terms and basic concepts, types of systems, intensive and extensive properties, state of system, thermodynamic processes, nature of heat and work, isothermal reversible and irreversible expansion works of an ideal gas, maximum work done in reversible expansion, internal energy, first law of thermodynamics, enthalpy of a system, molar heat capacities, Joule-Thomson Effect, adiabatic expansion of an ideal gas, spontaneous process, the Carnot cycle, derivation of entropy from Carnot cycle, entropy change in an irreversible process, entropy change for an ideal gas, Zeroth law of thermodynamics. The final part is concerned with nature of aromatic compounds, and benzene and its homologues.
In second semester, students will learn Structures of Ionic Compounds: close packing of spheres, interstitial sites, crystals, binary and ternary compounds; Basic Concepts of Chemical Kinetics: rate or velocity of a reaction, collision theory of reaction rate, factors affecting the reaction rate, rate law and rate constant, ionic and molecular reactions, molecularity of a reaction, order of a reaction, reaction of the first order, second order and third order, reversible or opposing reactions, reaction of higher order-explanation of their rarity on the bases of reaction mechanism, zero order reactions, determination of the order of a reaction, disturbing factors in the determination of order; and Chemical Equilibrium: reversible process, thermodynamic derivation of the equilibrium constant, the Le-Chatelier-Braun Principle, types of chemical equilibria, variation of equilibrium constant with temperature and pressure.
In first semester, student able to familiarize with 1) Aromatic Compounds: nature and structure, aromaticity and Hückel rule, and benzene and its homologues; 2) Natural Products and Heterocyclic Compounds: natural products and commercial medicines, phytochemicals of medicinal value in plants, heterocyclic compounds and alkaloids; and 3) Polyfunctional Compounds: polyhydric alcohols, dicarboxylic acids, hydroxy acids, keto acids, keto esters and diesters.
In second semester student will learn three main parts. The first parts deals with isomerism of organic compounds that includes classification of isomerism, constitutional structural isomerism, and conformational isomerism. Second part concerns with carbohydrates that includes nomenclature, monosaccharides, disaccharides and polysaccharides, formation of esters and ethers, formation of glycosides. The final part reveals natural and synthetic polymers and in this session students will get knowledge on general principle of polymer formation, polymerization, vinyl polymers, structure and stereochemistry of polymers.
Organometallic Chemistry: classification, bonding and synthetic methods, thermodynamic and kinetic stabilities, properties and reactions, catalytic reactions, stoichiometric reactions (insertion, oxidative-addition).
Complexes of Acceptor π Ligands: noble gas formulation, carbon monoxide complexes, nitric oxide complexes, donor complexes of group VB and group VIB ligands, cyanide complexes, and ligands having extended π systems.
Aim of the first part is to provide knowledge on theoretical concepts of organic chemistry that is essential to learn typical organic reactions further. In this part, students will learn what are meant by reactants and reactions, what types of reactions happen in organic compounds, factors that influencing a reaction, what are carbocations, carbanions, and radicals.
In second part, students are able to learn nucleophilic substitution reactions, factors affecting SN1 and SN2 reactions, elimination reaction, competition between Substitution and elimination.
Practical Aspects of Chemical Analysis: analysis of real samples, preparing samples for analysis, decomposing and dissolving the samples; Treatment of Analytical Data: significant figures, accuracy and precision, types of error in experimental data, statistical treatment of data; Gravimetric Analysis: general principles of gravimetric analysis, stoichiometry of gravimetric reaction, formation and properties of precipitates, drying and ignition of precipitates, organic precipitants, solubility equilibrium; Precipitation Titrations: requirements for precipitation titrations, titration curve for precipitation titrations, end points for Argentometric titrations, applications of standard silver nitrate solutions.
Fundamental Concepts: bioelements, important functional groups in biomolecules, the specific interactions of biomolecules depend on noncovalent bonds, properties of water; Biomolecules: carbohydrates, lipids, amino acids, nucleic acids; Enzymes: characteristics, nomenclature and classification, mechanism and types of enzyme action, enzyme catalysts activity, factors that affect enzyme activity, enzyme kinetic, enzyme units, enzyme inhibitor, enzyme parts list; Metabolism: what is cell, introduction to metabolism, intermediary metabolism, glycolysis, citric acid cycle; Molecular Genetics: Waston-Crick double helix, biosynthesis of DNA (replication), biosynthesis of RNA (transcription), biosynthesis of protein (translation), and mutation.
Nonaqueous Solvents Systems: classification of solvents, general properties of ionising solvents, liquid ammonia as solvent, liquid sulphur dioxide as solvent, liquid hydrogen fluoride, liquid hydrogen cyanide, acetic acid, and other non-aqueous solvents.
Theories of the Coordinate Bond in Metal Complexes: valence bond theory, crystal field theory, ligand field theory, molecular orbital theory, and stability of complex ions.
Stereochemistry: stereoisomers, conformational and configurational isomers, prediction of enantiomerism, naming enantiomers by using R, S system of nomenclature, optical activity, specification of configuration for more than one chiral centre, diastereomers, meso compounds, separation of enantiomers, and resolution of a racemic modification.
Amino acids, Peptides, Proteins and Nucleic Acids: structures of amino acids, dipolar structure of amino acids, isoelectric point, separation of amino acids, synthesis of a-amino acids, reactions of amino acids, resolution of racemic mixtures of amino acids, peptides, determination of peptide structure, synthesis of peptides, classification of proteins, protein structures, determination the primary structure of a protein, denaturation of protein, nucleic acids, the structure of DNA and RNA, and hydrolysis of nucleic acid.
Acid Base Equilibria and Titration: fundamental concept of acidity and basicity, equilibrium calculations for solutions of acids and bases, acid-base titration, and titration curves; Equilibria in Oxidation Reduction Systems: oxidation-reduction equilibria, half-reactions, fundamentals of electrochemistry, schematic representation of electrochemical cells, electrode potential or relative half-cell potential, effect of concentration on electrode potentials; Potentiometric Methods: electrode systems, inert electrodes, measurement of cell emf, the potentiometer, potentiometric titrations, reference electrode, indicator electrodes and salt bridge and liquid junction potentials
In second semester students are able to learn and get experience in separation techniques of organic compounds such as solvent extraction methods and chromatographic separation techniques.
Stability of Isotopes: nuclear structure and stability, radioactivity and nuclear decay, discovery of isotopes, nuclear reaction, nuclear models, analysis of radioactive environmental sample, and health and safety aspects; Symmetry and Point Groups: symmetry operations and symmetry elements, inverse operations, groups and their basic properties, point groups, systematic classification of molecules into point groups, and matrices; Toxicological Effects of Mercury and Arsenic Compounds: sources, physical and chemical properties, compounds of mercury and arsenic, health effects, risk assessment, preventive and remedial measures.
In second semester, Fundamental Aspects of Solid State Chemistry: experimental evidence on structure, structure and properties, and structure and properties of transition metal oxides; and Group Theory and its Applications: representation of groups, reducible and irreducible representation, some important reducible representation, group theory and vibrational spectroscopy, some further aspects of vibrational spectroscopy, some applications of group theory in bonding would be learnt.
In second semester student will learn Quantum Chemistry that reveals (i) Atoms: Electronic Structure and Spectra describing on the lowest energy level of the helium atom, spin and antisymmetric wave functions, electronic configurations of atoms and the periodic table, interpretation of atomic spectra; and (ii) Diatomic Molecule: Bonding and Electronic Spectra Ionic Interactions that describes separation of nuclear and electronic motion, the hydrogen molecular ion, homonuclear and heteronuclear diatomic molecules and electronic spectroscopy. In addition, students will learn Material Science that reveals (i) The Geometry of Nanoscale Carbon and Fullerenes depicting bonding, dimensionality, topology, curvature, energetics, kinetics, other rings, holes, families of fullerenes: from C60 to TNTs, reactivity, potential applications and (ii) Characterization and Properties of Nanomaterials presenting introduction, structural characterization, chemical characterization, physical properties of nanomaterials, electrical conductivity, ferroelectrics and dielectrics, superparamagnetism.
Chem 5209 composed of three main parts, namely, Synthesis of Organic Compounds, Chromatographic Separation Techniques, and Organometallic Compounds. The first part includes multistep organic synthesis, functional group introduction, removal and interconversion, and retrosynthetic analysis (disconnections). The second part, students can learn partition chromatography, thin layer and paper chromatography, adsorption chromatography and column chromatography. The final part of this module concerns with Organometallic Compounds that describes nomenclature, carbon-metal bonds, preparation of organolithium compound, preparation of magnesium compound, Grignard reagent, synthesis of alcohol using Grignard or Organolithium reagents, synthesis of acetylenic alcohols, retrosynthetic analysis, preparation of tertiary alcohol from esters and Grignard reagents, alkane synthesis using organocopper reagents, an organozinc reagent for cyclopropane synthesis, carbenes and carbenoids, and transition metal organometallic compound.
Later module composed of three parts, namely, Optical Methods, Continuous Automatic Instrumentation for Process Application, and ORD and CD. The details descriptions of these courses are as follow. Optical Methods (Emission, Absorption, Fluorescence): fundamentals of spectrophotometry, spectroscopic instruments and analysis; Continuous Automatic Instrumentation for Process Application: autoanalyzer, process analyzer; and Optical Rotatory Dispersion and Circular Dichroism: property of light wave, optical rotation, ORD, CD, relationship of ORD and CD, the physical basis of optical rotation and CD, Usefulness of CD.
In second semester, students will learn Methods of Production of Isotopes and Nuclear Energy concerning with production of radioisotopes, production of neutron-excess radioisotopes, production of neutron- deficient radioisotopes, generator produced radioisotopes, activation analysis, radio isotopic purity, natural production of radioisotopes, basic principles of chain-reacting system, reactors and their uses, reactor- associated problems, and controlled-thermonuclear reactions.
These modules concern with inorganic chemistry and nuclear chemistry including a wide range of topics in transition elements and the electronic structures of their compounds; inorganic reaction mechanisms of complexes; homogeneous and heterogeneous catalytic reactions of organometallic compounds; bioinorganic chemistry, solid state chemistry, advanced nuclear chemistry and selected topics.